Abstract
Background: In a large, pooled analysis from the InterLymph Consortium, autoimmune diseases classified as B-cell activating were associated with an increased risk of lymphoma, particularly diffuse large B-cell (DLBCL) and marginal zone (MZL) lymphoma, while autoimmune diseases classified as T-cell activating were only associated with risk of T-cell lymphoma (TCL). Because the role of autoimmune disease in lymphoma prognosis is less well studied, we evaluated lymphoma subtype-specific outcomes by autoimmune history overall as well as by B-cell activating and T-cell activating autoimmune disease groupings.
Methods: To evaluate these associations, we used Mayo Clinic cases enrolled in the University of Iowa/Mayo Clinic SPORE Molecular Epidemiology Resource (MER), a prospective cohort study. Participants enrolled at Mayo Clinic with self-reported risk factor data on 8 autoimmune diseases were eligible, including 736 DLBCL, 703 follicular lymphoma (FL), 302 MZL, 193 mantle cell lymphoma (MCL), 297 Hodgkin lymphoma (HL), and 186 TCL. Autoimmune conditions were categorized as either B-cell activating (rheumatoid arthritis [RA], Sjögren syndrome, systemic lupus, and Hashimoto thyroiditis) or T-cell activating (celiac disease, Crohn's, ulcerative colitis, and polymyositis/dermatomyositis) according to the InterLymph classification. Clinical and treatment data were abstracted, and participants were prospectively followed for outcomes. We defined event-free survival (EFS) as time from diagnosis to progression/relapse, re-treatment, or death, and overall survival (OS) as time from diagnosis to death due to any cause. We used Cox regression analysis to estimate hazard ratios (HR) and 95% confidence intervals (CI), adjusting for the following variables: sex, International Prognostic Index (for DLBCL, MZL and TCL); Mantle Cell International Prognostic Index (for MCL); International Prognostic Score (for HL); Follicular Lymphoma International Prognostic Index, FL grade III, and rituximab-based therapy (for FL); and immunochemotherapy treatment (for DLBCL).
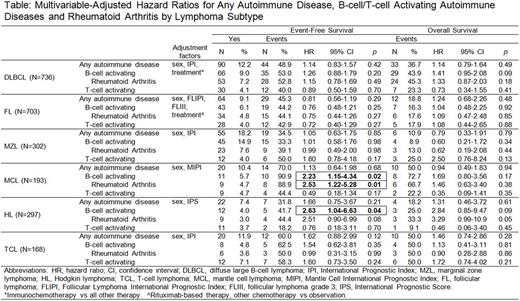
Results: The prevalence of any of the 8 self-reported autoimmune diseases varied across subtypes, and was highest in MZL (18.2%), followed by TCL (11.9%), MCL (10.4%), DLBCL (12.2%), FL (9.1%) and HL (7.4%). The prevalence of B-cell activating vs. T-cell activating autoimmune disease was higher in DLBCL, FL, MZL, and MCL, similar in HL, and lower in TCL. Although history of any autoimmune disease did not associate with EFS or OS for any lymphoma subtype, there was a positive association of B-cell activating autoimmune disease with inferior EFS in MCL (HR=2.23, 95% CI 1.15-4.34) and HL (HR=2.63, 95% CI 1.04-6.63), which was largely driven by RA (Table). T-cell activating autoimmune disease did not associate with EFS in any subtype, although there were generally a small number of events for this exposure. While there were no statistically significant associations for any of the autoimmune variables with OS, relationships between B-cell activating autoimmune disease and inferior OS in MCL (HR=1.69, 95% CI 0.80-3.56) and HL (HR=2.84, 95% CI 0.85-9.47) appeared to parallel the EFS associations observed in these subtypes.
Conclusions: Overall, we found little evidence for an important role of autoimmune disease in lymphoma outcomes, although our results suggest inferior EFS and perhaps OS for MCL and HL with a history of B-cell activating autoimmune disease, particularly RA. Further investigation and validation is needed for self-reported autoimmune diseases, and larger sample sizes are also warranted to fully understand the role of these not-uncommon diseases in the management of lymphoma patients.
Witzig: Novartis: Research Funding; Acerta: Research Funding; Kura: Research Funding; Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding. Nowakowski: pharmacyclics: Consultancy; Nanostring: Research Funding; Bayer: Consultancy, Research Funding; Genetech: Consultancy, Research Funding; Morphosys: Consultancy, Research Funding; Abbvie: Consultancy; celgene: Consultancy, Research Funding. Flowers: Janssen Pharmaceutical: Research Funding; Gilead: Consultancy; National Institutes Of Health: Research Funding; V Foundation: Research Funding; Millennium/Takeda: Research Funding; Burroughs Welcome Fund: Research Funding; Celgene: Consultancy, Research Funding; TG Therapeutics: Research Funding; Eastern Cooperative Oncology Group: Research Funding; National Cancer Institute: Research Funding; Seattle Genetics: Consultancy; Acerta: Research Funding; Onyx: Research Funding; Infinity: Research Funding; Prime Oncology: Research Funding; Educational Concepts: Research Funding; Clinical Care Options: Research Funding; Genentech/Roche: Consultancy, Research Funding; Abbvie: Consultancy, Research Funding; Spectrum: Consultancy; OptumRx: Consultancy; Bayer: Consultancy; Research to Practice: Research Funding; Pharmacyclics LLC, an AbbVie Company: Research Funding. Cerhan: Janssen: Other: Scientific Advisory Board (REMICADELYM4001); Janssen: Other: Multiple Myeloma Registry Steering .
Author notes
Asterisk with author names denotes non-ASH members.